What Is Medical Surface and Coating Testing?
Medical device surface and coating testing evaluates the suitability of the surface state and coatings chosen for the medical devices. It is a crucial process in the development, approval, and maintenance of medical devices. It ensures that the materials and coatings used in these devices are safe, durable, biocompatible and effective in their intended medical applications.
At Applus+ Laboratories, we carry out surface and coating testing on material samples as well as on finished implants and surgical instruments to ensure an increasingly reliable product for the patient. This is part of our comprehensive and dedicated service for medical device testing.
What Medical Device Surface and Coating Testing Services Do We Offer?
We offer comprehensive surface morphological topographical and physical characterisation of your medical device to support you and bring deep insight in the material comprehension, potential change and change control monitoring, reverse engineering, and audits.
Physical, Morphological and topographical (PMT) characterisation according to ISO 10993-19
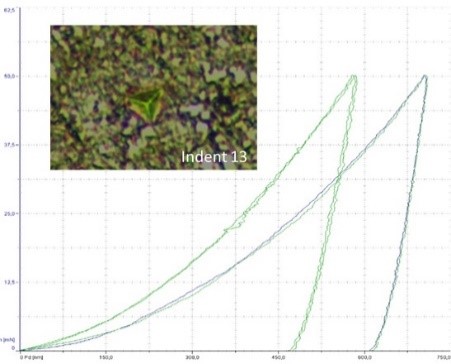
These analyses are coupled with a comprehensive assessment of the surface state, including topography, wettability, roughness, and wear resistance. These tests allow verification of the quality of the interface and adhesion between coatings and medical devices (to be linked with surface and coating sheet).
We offer testing services for both the materials used in implants, such as metals, ceramics and organic materials, as well as the coatings that are applied to them. We test against a range of different international standards that ensures they are fit to be placed into the human body.

- Surface energy and wettability according to ISO 19403-2: This determines wettability and the compatibility of the surface with a coating or adhesives.
- Surface Roughness according to ISO 21920
- Coating thickness according to ISO 2360: This standard evaluates the thickness of non-metallic coatings on metallic
- Particle Size Characterisation: We use diffraction, laser granulometry, and high-resolution microscopy to identify particle sizes to control the release rate of coatings on joint implants and the presence of contaminants in medical devices.
- SEM Microscopy with Elemental Analysis: We employ Scanning Electron Microscopy (SEM) which provides high resolution images of the surface morphology of medical device coatings and presence of particles on the surface. This is coupled with elemental analysis that provides the element composition of the structure.
- Chemical analysis by FTIR spectroscopy to evaluate the chemical nature of the surface of the device
- Surface topography and roughness with a stylus profilometer and an optical high-resolution microscope
Physical and chemical characterisation of coating according to specific standard
On Ceramic coating
- ISO 13779-1 (joint implants): This standard requires testing ceramic hydroxyapatite for its chemical composition to ensure that it is suitable to be implanted into the human body as well as the physical properties to check for its density and porousness. The standard also includes testing ceramic hydroxyapatite for its biocompatibility as well as its mechanical strength.
- ASTM F1044: This standard assesses the shear strength of calcium phosphate and metallic coatings, focusing on their ability to adhere to the medical device's surface. It specifically tests whether the coatings can resist forces that might cause them to slide across the substrate.
- ASTM F1147: This test measures the tensile strength of coatings to determine how well they adhere under tension. It ensures that the coatings remain securely attached and intact even when stretched.
- ISO 13779-4: This standard evaluates the adhesive strength of hydroxyapatite coatings on metallic implants. The test ensures that the coatings maintain their position and structural integrity under the normal stresses experienced within the body.
On all surface treatment and coating
We can measure the coating thickness on non-metallic substrates:
- ISO 13320: This standard evaluates the size of particles (powder or in a liquid) by a granulometry measurement.
- ISO 1518 – ISO 15184: These standards evaluate the scratch resistance and hardness of coatings.
- ISO 2409: This standard evaluates the adhesion of coatings on a surface.
- ISO 4624: This standard evaluates the adhesion of coatings on a surface.
Environmental resistance of coating and surface
Taking into consideration the external environment to evaluate the risk of evolution of the medical device either under mechanical or physical stress such as wear, impact or even fatigue or under chemical stress such as sterilisation or contact with a cleaning product, a clinical fluid, is crucial to anticipate safety issues that could rise from the use of the device.
- Corrosion resistance according to ASTM F2129: Standard test method for conducting cyclic potentiodynamic polarisation measurements to determine the corrosion susceptibility of small implant devices
- Fatigue resistance according to ASTM F1160: This standard tests the fatigue resistance of coatings made from calcium phosphate or metallic composites on medical implants. It evaluates how these coatings perform under cyclic loading conditions, simulating the long-term wear and tear they would experience during use.
- Impact resistance according to ISO 6272: This standard evaluates the impact resistance of coatings.
- Surface wear resistance
- According to ASTM G99: This standard evaluates surface wear resistance, wear rate and friction coefficient of coatings.
- According to ASTM D4060: This standard evaluates the wear resistance of coatings.

What Are the Advantages of Medical Device Surface and Coating Testing?
There are a myriad of advantages of getting surface and coating testing performed on your medical devices. From improving the materials used to improving market access, here are the principal advantages:
- Ensuring Patient Safety: Undergoing the relevant testing for materials and coatings ensures that patients will receive implants that have been fully tested and that they are safe to be placed in the human body. This massively boosts patient confidence that they are receiving an implant that has undergone stringent testing.
- Boosting Innovation: Putting your medical device through its paces is a crucial step toward boosting innovation. As new and more efficient materials are being trialled for implants, having the relevant testing performed can help resolve weak points in the new materials and reveal whether they are good enough to be used. This means that more innovative materials can be brought to the market quicker.
- Increasing Market Access: Complying with the international standards on materials and coatings can vastly increase your global market access. Being certified in complying with these standards can allow you to enter different marketplaces so you can sell your medical devices all over the world. In addition, undergoing testing can also speed up your product to market.
Why Choose Applus+ Laboratories for Medical Surface and Coating Testing?
Opting for Applus+ Laboratories for your surface and coating testing aligns you with a leader in the field of medical device testing.
We provide ASTM and ISO-compliant services to ensure that your surface and coating testing meet the highest standards of assessment for fatigue and adhesion. With a broad spectrum of testing capabilities and a focus on customer service, we are perfectly suited to handle all your surface and coating testing.
Applus+ Laboratories is committed to being your one-stop shop for medical device testing, offering a complete catalogue of services designed to reduce your product’s time to market. Our services include:
- Development testing and suggestions for improvements
- Testing throughout the product lifecycle
- Qualification of products and processes, along with batch release testing
Present in various countries, we are equipped to offer our testing services globally, ensuring access to top-notch surface and coating testing in multiple countries.
Choose Applus+ Laboratories as your dependable partner for surface and coating testing. We are ready to support your endeavours with our comprehensive services and expert advice.